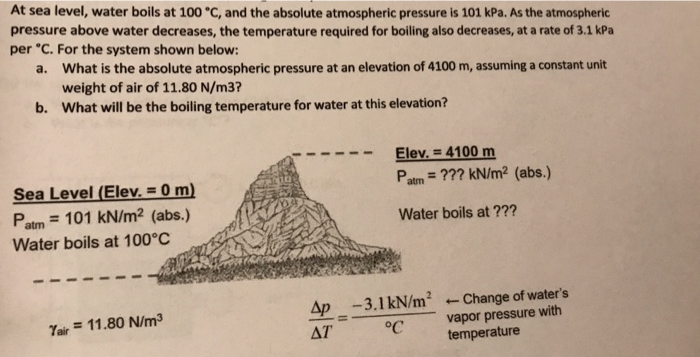
Sep 30, 20221. First, we need to find the amount of energy required to raise the temperature of the water to its boiling point (100°C). We can use the specific heat formula for this: Q = mcΔT where Q is the energy in joules, m is the mass of the water in grams, c is the specific heat capacity of water (4.18 J/g°C), and ΔT is the change in temperature.
Solved At sea level, water boils at 100 °C, and the absolute | Chegg.com
With a rough container, and a natural water source, which contains impurities, there is enough “real estate” for the process to occur at the STP boiling point of 100°C. With purified water in a smooth container, the water is able to absorb more heat energy than normal, and not begin reacting until the heat is too great, or a nucleation site is

Source Image: www.youtube.com
Download Image
The normal boiling point of water is 100 °C or 212 °F. Changes in elevation affect boiling point because they affect atmospheric pressure. The normal boiling point of water is 100 °C, 212 °F, or 373.1 K. The “normal” refers to sea level or an elevation of 0 meters or feet. But, the boiling point of water changes with elevation.

Source Image: www.numerade.com
Download Image
RECIPE ⬇️🍵🍪🍦🍯 1 tsp matcha milk of choice 1 tsp vanilla paste (yo… | TikTok Water will “boil” (evaporate) at 20⁰ C as long as the contribution of the water vapor to the total pressure of the atmosphere (about 1 atm) is small, say < 0.2 atm. Water boils at 100⁰ C at 1 atm pressure is correct (water boils at 20⁰ C at about 0.2 atm pressure). This boiling we do in the kitchen kettle is the bubble that forms at the

Source Image: www.quora.com
Download Image
Boiling 21.1 G Of Water At 100 C
Water will “boil” (evaporate) at 20⁰ C as long as the contribution of the water vapor to the total pressure of the atmosphere (about 1 atm) is small, say < 0.2 atm. Water boils at 100⁰ C at 1 atm pressure is correct (water boils at 20⁰ C at about 0.2 atm pressure). This boiling we do in the kitchen kettle is the bubble that forms at the Jan 18, 2024Hopefully, you see how the water heater BTU is related to this now! Latent heat, on the contrary, doesn’t refer to a change in temperature but a phase. This is the amount of heat required to turn, e.g., a liquid of some mass into a gas – you could think of what happens to water at 100°C when it becomes steam. In this case, the units are J/kg.
The boiling point of water is 100°C at 1.01325 bar. How does water evaporate from water bodies when the temperature is approximately 30°C at 1 bar? – Quora
Jul 31, 202321. How much heat in BTUs is required to raise the temperature of 1 lb of water from freezing to boiling point? … The energy transferred when 1g of boiling water at 100°C condenses to water at 100°C is equal to the latent heat of vaporization of water, which is approximately 2260 J/g. SOLVED: How many joules are required for boiling 21.1g of water at 100°c

Source Image: www.numerade.com
Download Image
If water boils at 100 degrees Celsius, then what will be the temperature of water vapour? – Quora Jul 31, 202321. How much heat in BTUs is required to raise the temperature of 1 lb of water from freezing to boiling point? … The energy transferred when 1g of boiling water at 100°C condenses to water at 100°C is equal to the latent heat of vaporization of water, which is approximately 2260 J/g.
Source Image: www.quora.com
Download Image
Solved At sea level, water boils at 100 °C, and the absolute | Chegg.com Sep 30, 20221. First, we need to find the amount of energy required to raise the temperature of the water to its boiling point (100°C). We can use the specific heat formula for this: Q = mcΔT where Q is the energy in joules, m is the mass of the water in grams, c is the specific heat capacity of water (4.18 J/g°C), and ΔT is the change in temperature.
Source Image: www.chegg.com
Download Image
RECIPE ⬇️🍵🍪🍦🍯 1 tsp matcha milk of choice 1 tsp vanilla paste (yo… | TikTok The normal boiling point of water is 100 °C or 212 °F. Changes in elevation affect boiling point because they affect atmospheric pressure. The normal boiling point of water is 100 °C, 212 °F, or 373.1 K. The “normal” refers to sea level or an elevation of 0 meters or feet. But, the boiling point of water changes with elevation.

Source Image: www.tiktok.com
Download Image
Highway to English – Water at 100 degrees celsius. 1) boils 2) boil 3) is boiling 4) boiling CLICK to LIKE and SHARE! | Facebook At these conditions, you can drill a hole into container and store water at air pressure. But when you heat water to tempertature over 100°C, it starts to boil, increasing pressure in contailner. If you drill a hole, water will start to evaporate and that’ll cause temperature drop. If you try to maintain constant temperature, after some time

Source Image: www.facebook.com
Download Image
Water boils at 212°F or 100°C and ice melts at 32°F or 0°C . If the temperature of a pot of water is 40°C, what is the temperature of the pot of Water will “boil” (evaporate) at 20⁰ C as long as the contribution of the water vapor to the total pressure of the atmosphere (about 1 atm) is small, say < 0.2 atm. Water boils at 100⁰ C at 1 atm pressure is correct (water boils at 20⁰ C at about 0.2 atm pressure). This boiling we do in the kitchen kettle is the bubble that forms at the
Source Image: www.quora.com
Download Image
Does Boiling Water Keep Getting Hotter? Jan 18, 2024Hopefully, you see how the water heater BTU is related to this now! Latent heat, on the contrary, doesn’t refer to a change in temperature but a phase. This is the amount of heat required to turn, e.g., a liquid of some mass into a gas – you could think of what happens to water at 100°C when it becomes steam. In this case, the units are J/kg.

Source Image: sciencenotes.org
Download Image
If water boils at 100 degrees Celsius, then what will be the temperature of water vapour? – Quora
Does Boiling Water Keep Getting Hotter? With a rough container, and a natural water source, which contains impurities, there is enough “real estate” for the process to occur at the STP boiling point of 100°C. With purified water in a smooth container, the water is able to absorb more heat energy than normal, and not begin reacting until the heat is too great, or a nucleation site is
RECIPE ⬇️🍵🍪🍦🍯 1 tsp matcha milk of choice 1 tsp vanilla paste (yo… | TikTok Water boils at 212°F or 100°C and ice melts at 32°F or 0°C . If the temperature of a pot of water is 40°C, what is the temperature of the pot of At these conditions, you can drill a hole into container and store water at air pressure. But when you heat water to tempertature over 100°C, it starts to boil, increasing pressure in contailner. If you drill a hole, water will start to evaporate and that’ll cause temperature drop. If you try to maintain constant temperature, after some time