The first ionization energies of the transition metals are somewhat similar to one another, as are those of the lanthanides. Ionization energies increase from left to right across each row, with discrepancies occurring at ns 2 np 1 (group 13), ns 2 np 4 (group 16), and ns 2 (n − 1)d 10 (group 12). First ionization energies generally decrease
Solution] Periodic Trends: Ionization Energy | Wizeprep
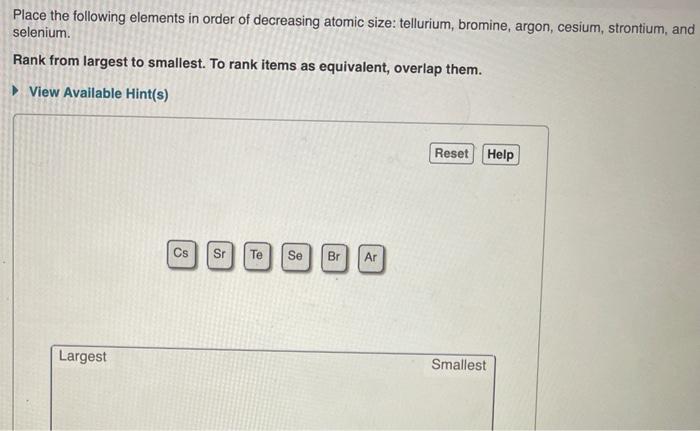
Find step-by-step Chemistry solutions and your answer to the following textbook question: Arrange the following elements in order of decreasing first ionization energy: $$ \ce S, Ca, F, Rb, and Si $$ Rank from largest to smallest. To rank items as equivalent, overlap them..
Source Image: chegg.com
Download Image
Refer to LibreText Section 3.2.4: Variation in Ionization Energies. 3. Summarize the trend in the periodic table . Ionization Energies: The ionization energies increase from left to right, and decrease from top to bottom. 5. Find the elements on the periodic table and arrange them based on the trend of increasing ionization energies.

Source Image: slideplayer.com
Download Image
Electronegativity – Definition, Periodic Trends, Effect on Bonding, FAQs on Electronegativity
Chemistry Chemistry questions and answers 12) Rank the following in terms of decreasing first ionization energies? A) Ne > O> N > Be > B B) Ne>N> O> B> Be C) Ne > O> N > B> Be D) B > Be> O> N > Ne E) Ne > N> O > Be > B This problem has been solved! You’ll get a detailed solution from a subject matter expert that helps you learn core concepts.
Source Image: quora.com
Download Image
Rank The Following In Terms Of Decreasing First Ionization Energies
Chemistry Chemistry questions and answers 12) Rank the following in terms of decreasing first ionization energies? A) Ne > O> N > Be > B B) Ne>N> O> B> Be C) Ne > O> N > B> Be D) B > Be> O> N > Ne E) Ne > N> O > Be > B This problem has been solved! You’ll get a detailed solution from a subject matter expert that helps you learn core concepts.
Rank the following in terms of decreasing first ionization energies? O Ne > N>O> B> Be O B> Be > O> N > Ne O Ne > N>O> Be > B O Ne > 0>N> Be > B Ne > 0>N>B> Be BUY Introductory Chemistry: An Active Learning Approach 6th Edition ISBN: 9781305079250 Author: Mark S. Cracolice, Ed Peters Publisher: Cengage Learning expand_more
How to arrange the following in the order of decreasing ionization energy, Li, Na, C, O, and F – Quora
Rank each of the elements in order of decreasing ionization energy: S, Cl, Br? (Justify) Chemistry The Periodic Table Periodic Trends in Ionization Energy 1 Answer zhirou Jun 11, 2018 Cl > Br > S. Explanation: Ionization energy is the energy needed to remove one electron from an atom in the gaseous state.
Utilizing Machine Learning and Diode Physics to Investigate the Effects of Stoichiometry on Photovoltaic Performance in Sequentially Processed Perovskite Solar Cells | ACS Omega

Source Image: pubs.acs.org
Download Image
Periodic Table Trends | Atomic and Ionic Radii, Ionisation Enthalpy
Rank each of the elements in order of decreasing ionization energy: S, Cl, Br? (Justify) Chemistry The Periodic Table Periodic Trends in Ionization Energy 1 Answer zhirou Jun 11, 2018 Cl > Br > S. Explanation: Ionization energy is the energy needed to remove one electron from an atom in the gaseous state.

Source Image: byjus.com
Download Image
Solution] Periodic Trends: Ionization Energy | Wizeprep
The first ionization energies of the transition metals are somewhat similar to one another, as are those of the lanthanides. Ionization energies increase from left to right across each row, with discrepancies occurring at ns 2 np 1 (group 13), ns 2 np 4 (group 16), and ns 2 (n − 1)d 10 (group 12). First ionization energies generally decrease
![Solution] Periodic Trends: Ionization Energy | Wizeprep](https://d3rw207pwvlq3a.cloudfront.net/attachments/000/064/596/original/image.png?1568856829)
Source Image: wizeprep.com
Download Image
Electronegativity – Definition, Periodic Trends, Effect on Bonding, FAQs on Electronegativity
Refer to LibreText Section 3.2.4: Variation in Ionization Energies. 3. Summarize the trend in the periodic table . Ionization Energies: The ionization energies increase from left to right, and decrease from top to bottom. 5. Find the elements on the periodic table and arrange them based on the trend of increasing ionization energies.

Source Image: byjus.com
Download Image
How To Rank Ionization Energy of Elements – YouTube
Study with Quizlet and memorize flashcards containing terms like In which direction on the periodic table does metallic character increase? A) Down and to the right. … Rank the following atoms in order of decreasing first ionization energies (i.e., highest to lowest): Li, Be, Ba, F.

Source Image: m.youtube.com
Download Image
Rank the following in order of decreasing ionization energy. Cl, F, Ne^+, S, S^- | Homework.Study.com
Chemistry Chemistry questions and answers 12) Rank the following in terms of decreasing first ionization energies? A) Ne > O> N > Be > B B) Ne>N> O> B> Be C) Ne > O> N > B> Be D) B > Be> O> N > Ne E) Ne > N> O > Be > B This problem has been solved! You’ll get a detailed solution from a subject matter expert that helps you learn core concepts.

Source Image: homework.study.com
Download Image
Periodic Trends – MCAT Physical
Rank the following in terms of decreasing first ionization energies? O Ne > N>O> B> Be O B> Be > O> N > Ne O Ne > N>O> Be > B O Ne > 0>N> Be > B Ne > 0>N>B> Be BUY Introductory Chemistry: An Active Learning Approach 6th Edition ISBN: 9781305079250 Author: Mark S. Cracolice, Ed Peters Publisher: Cengage Learning expand_more

Source Image: varsitytutors.com
Download Image
Periodic Table Trends | Atomic and Ionic Radii, Ionisation Enthalpy
Periodic Trends – MCAT Physical
Find step-by-step Chemistry solutions and your answer to the following textbook question: Arrange the following elements in order of decreasing first ionization energy: $$ \ce S, Ca, F, Rb, and Si $$ Rank from largest to smallest. To rank items as equivalent, overlap them..
Electronegativity – Definition, Periodic Trends, Effect on Bonding, FAQs on Electronegativity Rank the following in order of decreasing ionization energy. Cl, F, Ne^+, S, S^- | Homework.Study.com
Study with Quizlet and memorize flashcards containing terms like In which direction on the periodic table does metallic character increase? A) Down and to the right. … Rank the following atoms in order of decreasing first ionization energies (i.e., highest to lowest): Li, Be, Ba, F.