Jun 27, 2022Beginning Chemistry (Ball) 9: Chemical Bonds
Which is the correct Lewis structure of nitrite ion, NO2-? – ppt download
The strength of ionic bonding depends on the magnitude of the charges and the sizes of the ions. 10.3: Lewis Structures of Ionic Compounds- Electrons Transferred is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. The tendency to form species that have eight electrons in the valence shell is called

Source Image: quizlet.com
Download Image
Dec 10, 2023Answer Using Lewis Electron Structures to Explain Stoichiometry Formal Charges Example 8.2.2: The Ammonium Ion Strategy: Solution: Exercise 8.2.2 Answer Using Formal Charges to Distinguish Viable Lewis Structures Learning Objectives To use Lewis dot symbols to explain the stoichiometry of a compound

Source Image: toppr.com
Download Image
Solution] Resonance Structure, Oxidation State | Wizeprep
Draw Lewis dot structures for two hydrogen atoms and one oxygen atom. Attempt to arrange these three atoms so that they are sharing electrons. A finished “correct” structure should have every atom in the structure, once the sharing arrangements are made, with an electron arrangement that could be seen as “complete” or a “full shell.”
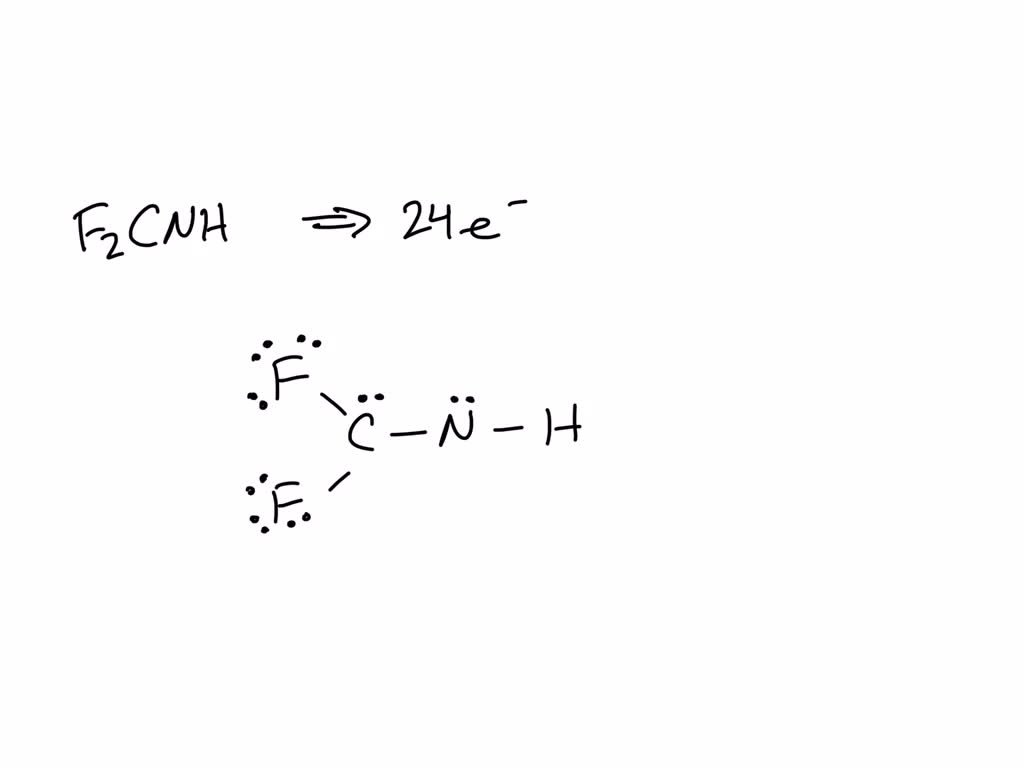
Source Image: numerade.com
Download Image
Select The Atoms Or Ions With Valid Lewis Dot Structures
Draw Lewis dot structures for two hydrogen atoms and one oxygen atom. Attempt to arrange these three atoms so that they are sharing electrons. A finished “correct” structure should have every atom in the structure, once the sharing arrangements are made, with an electron arrangement that could be seen as “complete” or a “full shell.”
Feb 22, 2022Answer 3-: This means that the atom or ion has gained 3 electrons, so we need to add 3 to the number of valence electrons for the neutral atom. For example, if the neutral atom is oxygen (O), which has 6 valence electrons, the 3- ion would have 6 + 3 = 9 valence electrons.
SOLVED: Write Lewis structures for the following molecules or ions. (Assign lone pairs, radical electrons, and atomic charges where appropriate.) F2CNH
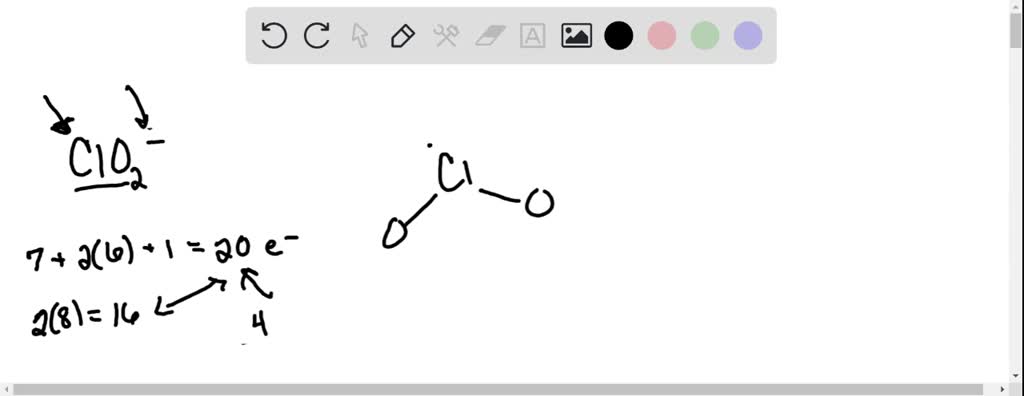
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a “skeleton structure.”.
SOLVED: Draw a valid Lewis structure for the ClO2- and and give the electron and molecular geometry and hybridization for the central Cl atom.
Source Image: numerade.com
Download Image
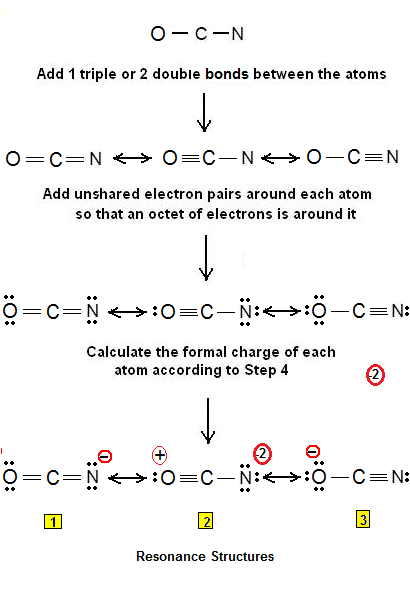
What is the lewis structure for OCN-? | Socratic
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a “skeleton structure.”.
Source Image: socratic.org
Download Image
Which is the correct Lewis structure of nitrite ion, NO2-? – ppt download
Jun 27, 2022Beginning Chemistry (Ball) 9: Chemical Bonds

Source Image: slideplayer.com
Download Image
Solution] Resonance Structure, Oxidation State | Wizeprep
Dec 10, 2023Answer Using Lewis Electron Structures to Explain Stoichiometry Formal Charges Example 8.2.2: The Ammonium Ion Strategy: Solution: Exercise 8.2.2 Answer Using Formal Charges to Distinguish Viable Lewis Structures Learning Objectives To use Lewis dot symbols to explain the stoichiometry of a compound
![Solution] Resonance Structure, Oxidation State | Wizeprep](https://d3rw207pwvlq3a.cloudfront.net/attachments/000/092/691/original/image.png?1575582060)
Source Image: wizeprep.com
Download Image
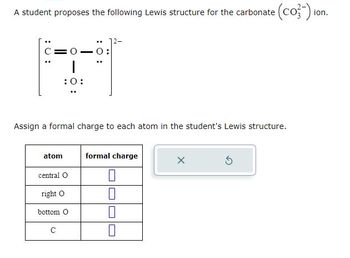
Answered: A student proposes the following Lewis… | bartleby
A Lewis electron dot symbol (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol
Source Image: bartleby.com
Download Image
CO2 Lewis Structure and Molecular Geometry – What’s Insight
Draw Lewis dot structures for two hydrogen atoms and one oxygen atom. Attempt to arrange these three atoms so that they are sharing electrons. A finished “correct” structure should have every atom in the structure, once the sharing arrangements are made, with an electron arrangement that could be seen as “complete” or a “full shell.”
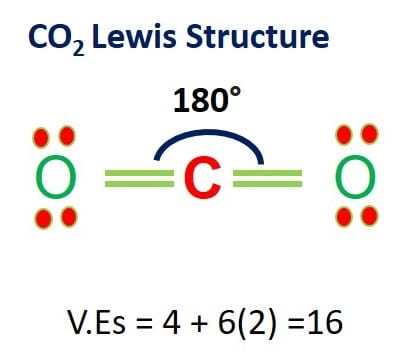
Source Image: whatsinsight.org
Download Image
Lewis Electron-Dot Structures | CK-12 Foundation
Feb 22, 2022Answer 3-: This means that the atom or ion has gained 3 electrons, so we need to add 3 to the number of valence electrons for the neutral atom. For example, if the neutral atom is oxygen (O), which has 6 valence electrons, the 3- ion would have 6 + 3 = 9 valence electrons.

Source Image: flexbooks.ck12.org
Download Image
What is the lewis structure for OCN-? | Socratic
Lewis Electron-Dot Structures | CK-12 Foundation
The strength of ionic bonding depends on the magnitude of the charges and the sizes of the ions. 10.3: Lewis Structures of Ionic Compounds- Electrons Transferred is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. The tendency to form species that have eight electrons in the valence shell is called
Solution] Resonance Structure, Oxidation State | Wizeprep CO2 Lewis Structure and Molecular Geometry – What’s Insight
A Lewis electron dot symbol (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol