Aug 13, 2023The corrosion process involves an oxidation-reduction reaction in which metallic iron is converted to Fe (OH) 3, a reddish-brown solid. Many metals dissolve through reactions of this type, which have the general form. metal+ acid → salt + hydrogen. Some of these reactions have important consequences.
Fluorogenic and Mitochondria-Localizable Probe Enables Selective Labeling and Imaging of Nitroreductase | Analytical Chemistry
B -. Answer. 10.8: Oxidation and Reduction in Organic Chemistry is shared under a CC BY-SA 4.0 license and was authored, remixed, and/or curated by Steven Farmer, Dietmar Kennepohl, Tim Soderberg, & Tim Soderberg. In organic chemistry, redox reactions look a little different. Electrons in an organic redox reaction often are transferred in the
Source Image: chegg.com
Download Image
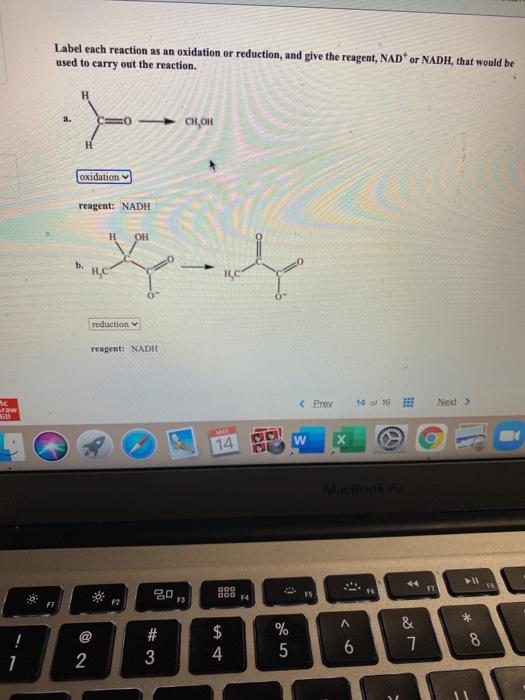
If they are, identify whether the molecule has been oxidized or reduced. (d) Calculate the oxidation numbers for each indicated atom in norethindrone, a steroid contraceptive. Classify each reaction as an oxidation, a reduction, or neither. (g) < of reaction> (h) < of reaction> (i) < of reaction>.

Source Image: pubs.acs.org
Download Image
SOLVED: Please write oxidation / neither / reduction. CHEM 222, Orgo 1 Oxidation or Reduction Recitation Worksheet Indicate whether each of the following reactions is oxidation, reduction, or neither. Explain your answers.
Aug 29, 2023An oxidation-reduction (redox) reaction is a type of chemical reaction that involves a transfer of electrons between two species. An oxidation-reduction reaction is any chemical reaction in which the oxidation number of a molecule, atom, or ion changes by gaining or losing an electron. Redox reactions are common and vital to some of the basic
Source Image: numerade.com
Download Image
Label Each Reaction As An Oxidation Reduction Or Neither
Aug 29, 2023An oxidation-reduction (redox) reaction is a type of chemical reaction that involves a transfer of electrons between two species. An oxidation-reduction reaction is any chemical reaction in which the oxidation number of a molecule, atom, or ion changes by gaining or losing an electron. Redox reactions are common and vital to some of the basic
Oxidation half-reaction: Al → Al3+ + 3e− Al → Al 3 + + 3 e −. To combine these two half reactions and cancel out all the electrons, we need to multiply the silver reduction reaction by 3: Now the equation is balanced, not only in terms of elements but also in terms of charge. The substance oxidized is the reactant that had undergone
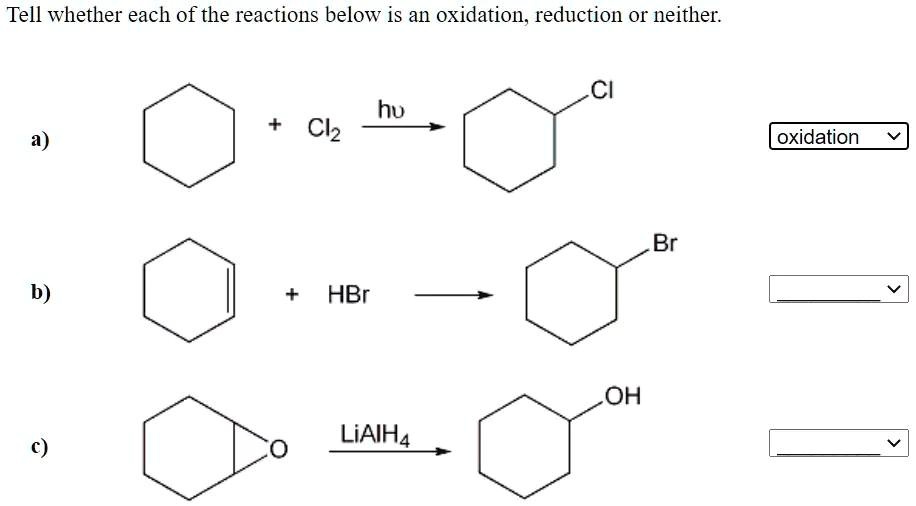
SOLVED: Text: Tell whether each of the reactions below is an oxidation, reduction, or neither: a) Cl b) Cl2 c) Br d) HBr e) OH f) LiAIH4
Figure 7.9.1 7.9. 1: Reaction between zinc and sulfur. Since the zinc is losing electrons in the reaction, it is being oxidized. The sulfur is gaining electrons and is thus being reduced. An oxidation-reduction reaction is a reaction that involves the full or partial transfer of electrons from one reactant to another.
Solved Redox) Label each reaction as oxidation, reduction or | Chegg.com
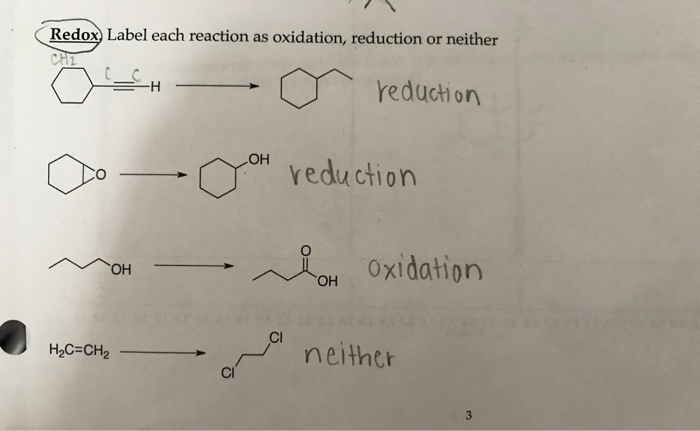
Source Image: chegg.com
Download Image
Classify each reaction as an oxidation, a reduction, or neither. … | Channels for Pearson+
Figure 7.9.1 7.9. 1: Reaction between zinc and sulfur. Since the zinc is losing electrons in the reaction, it is being oxidized. The sulfur is gaining electrons and is thus being reduced. An oxidation-reduction reaction is a reaction that involves the full or partial transfer of electrons from one reactant to another.

Source Image: pearson.com
Download Image
Fluorogenic and Mitochondria-Localizable Probe Enables Selective Labeling and Imaging of Nitroreductase | Analytical Chemistry
Aug 13, 2023The corrosion process involves an oxidation-reduction reaction in which metallic iron is converted to Fe (OH) 3, a reddish-brown solid. Many metals dissolve through reactions of this type, which have the general form. metal+ acid → salt + hydrogen. Some of these reactions have important consequences.

Source Image: pubs.acs.org
Download Image
SOLVED: Please write oxidation / neither / reduction. CHEM 222, Orgo 1 Oxidation or Reduction Recitation Worksheet Indicate whether each of the following reactions is oxidation, reduction, or neither. Explain your answers.
If they are, identify whether the molecule has been oxidized or reduced. (d) Calculate the oxidation numbers for each indicated atom in norethindrone, a steroid contraceptive. Classify each reaction as an oxidation, a reduction, or neither. (g) < of reaction> (h) < of reaction> (i) < of reaction>.
Source Image: numerade.com
Download Image
OsO4 (Osmium Tetroxide) for Dihydroxylation of Alkenes – Master Organic Chemistry
Jul 19, 202216.2: Oxidation and Reduction– Some Definitions. “Redox” is short for “oxidation and reduction“, two complimentary types of chemical reactions. The term oxidation originally referred to substances combining with oxygen, as happens when an iron bar rusts or a campfire log burns. We often refer to these two examples as corrosion and combustion.
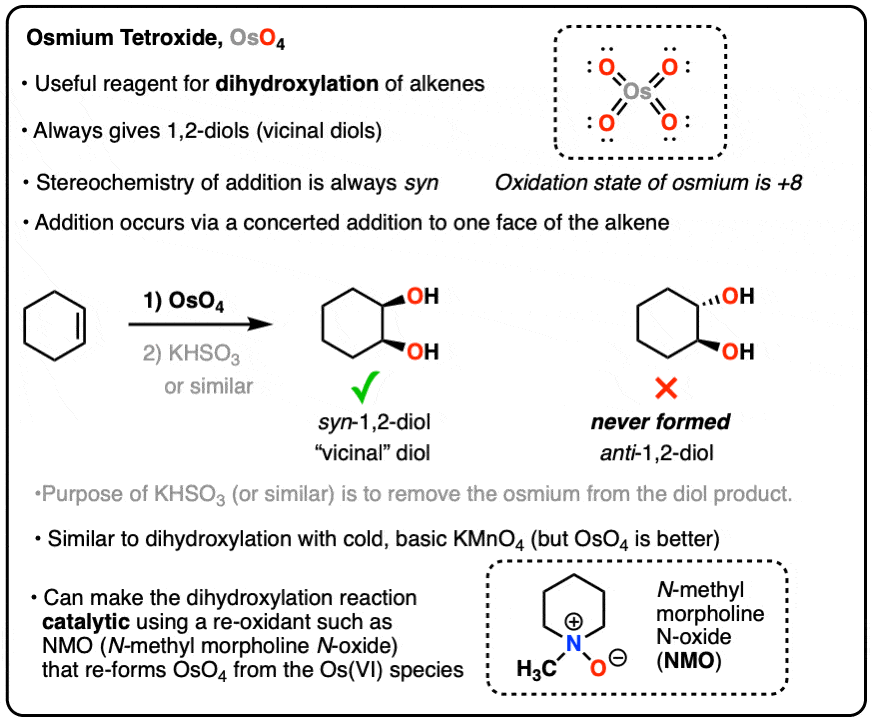
Source Image: masterorganicchemistry.com
Download Image
SOLVED: Label each reaction as oxidation, reduction, or neither.
Aug 29, 2023An oxidation-reduction (redox) reaction is a type of chemical reaction that involves a transfer of electrons between two species. An oxidation-reduction reaction is any chemical reaction in which the oxidation number of a molecule, atom, or ion changes by gaining or losing an electron. Redox reactions are common and vital to some of the basic
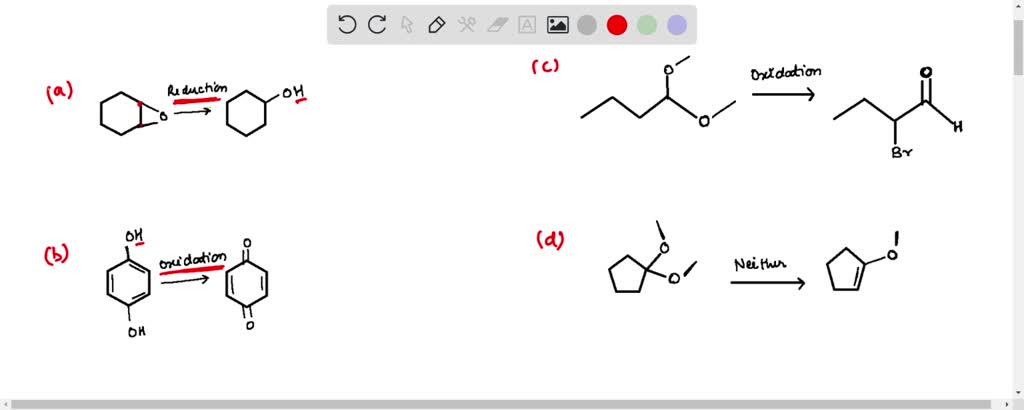
Source Image: numerade.com
Download Image
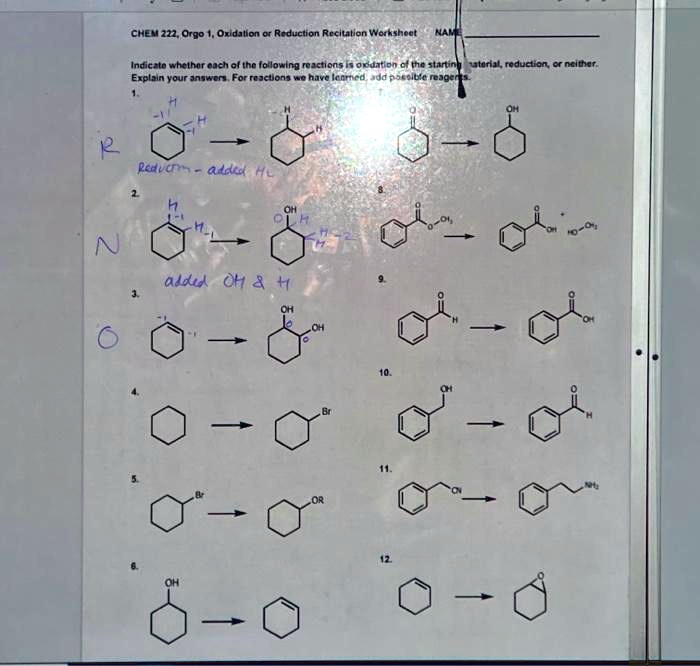
Solved Q 12.119: Is the reaction shown an oxidation, | Chegg.com
Oxidation half-reaction: Al → Al3+ + 3e− Al → Al 3 + + 3 e −. To combine these two half reactions and cancel out all the electrons, we need to multiply the silver reduction reaction by 3: Now the equation is balanced, not only in terms of elements but also in terms of charge. The substance oxidized is the reactant that had undergone
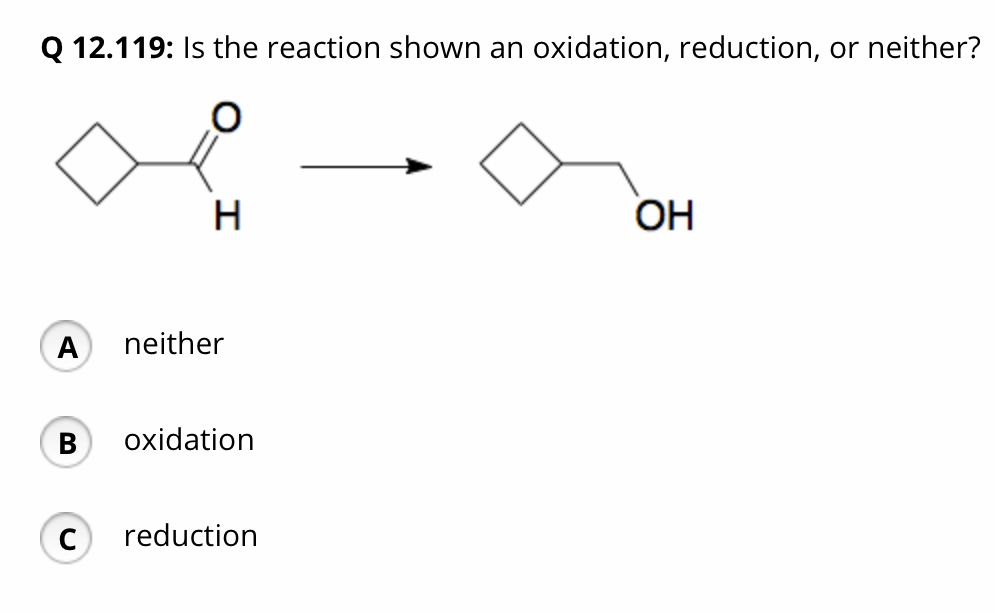
Source Image: chegg.com
Download Image
Classify each reaction as an oxidation, a reduction, or neither. … | Channels for Pearson+
Solved Q 12.119: Is the reaction shown an oxidation, | Chegg.com
B -. Answer. 10.8: Oxidation and Reduction in Organic Chemistry is shared under a CC BY-SA 4.0 license and was authored, remixed, and/or curated by Steven Farmer, Dietmar Kennepohl, Tim Soderberg, & Tim Soderberg. In organic chemistry, redox reactions look a little different. Electrons in an organic redox reaction often are transferred in the
SOLVED: Please write oxidation / neither / reduction. CHEM 222, Orgo 1 Oxidation or Reduction Recitation Worksheet Indicate whether each of the following reactions is oxidation, reduction, or neither. Explain your answers. SOLVED: Label each reaction as oxidation, reduction, or neither.
Jul 19, 202216.2: Oxidation and Reduction– Some Definitions. “Redox” is short for “oxidation and reduction“, two complimentary types of chemical reactions. The term oxidation originally referred to substances combining with oxygen, as happens when an iron bar rusts or a campfire log burns. We often refer to these two examples as corrosion and combustion.