Jan 30, 2023Recrystallization, also known as fractional crystallization, is a procedure for purifying an impure compound in a solvent. The method of purification is based on the principle that the solubility of most solids increases with increased temperature. This means that as temperature increases, the amount of solute that can be dissolved in a solvent
Solved orrect Question 10 0/0.5 pts Faster solid formation | Chegg.com
Dec 17, 2023Countdown to Giveaway Crystallization Of A Pure Compound Is Spontaneous Only Below December 17, 2023 Question: What does a negative sign for delta G indicate at constant temperature and pressure? Answer: The reaction must be spontaneous ==================================================

Source Image: numerade.com
Download Image
Crystallization of a pure compound is spontaneous only below 109. °C. This reaction is exothermic and proceeds faster at temperatures above 34. °C. conclusions AH is AS is AH is AS is AH is AS is (pick one) (pick one) (pick one) (pick one) (pick one) (pick one) Question

Source Image: scribd.com
Download Image
Fatty acid, triglyceride, and kinetic properties of milk fat fractions made by the combination of dry fractionation and short-pa A reactive crystallization could be performed for instance by adding a high pH solution to a low pH solution of a compound: the change in pH upon mixing the solutions reduces the solubility of the compound and crystallization can occur. A continuous reactive crystallization would have two solution feeds and a suspension outflow.

Source Image: yumpu.com
Download Image
Crystallization Of A Pure Compound Is Spontaneous Only Below
A reactive crystallization could be performed for instance by adding a high pH solution to a low pH solution of a compound: the change in pH upon mixing the solutions reduces the solubility of the compound and crystallization can occur. A continuous reactive crystallization would have two solution feeds and a suspension outflow. Please also note that some compounds simpl crystallize more easily than others. More rigid molecules are, as a rule, easier to crystalize. 8 Rigid, in this context, mean compounds that contain fewer bond capable of undergoing internal rotation, so that there are fewer possible conformers possible. Let us go through a recrystallization process, focusing on technical aspects and trouble shooting.
Sonic Warfare – La Ciudad TecniColor
3.4A: Purification. Crystallization is an excellent purification technique for solids because a crystal slowly forming from a saturated solution tends to selectively incorporate particles of the same type into its crystal structure (a model of a crystal lattice is in Figure 3.16a). A pure crystal is often slightly lower in energy than an impure ALEKS – Using the Conditions of Spontaneity to Deduce the Signs of ΔH and ΔS – YouTube

Source Image: m.youtube.com
Download Image
Consumer Psychology of Individuals (Chapter 1) – The Cambridge Handbook of Consumer Psychology 3.4A: Purification. Crystallization is an excellent purification technique for solids because a crystal slowly forming from a saturated solution tends to selectively incorporate particles of the same type into its crystal structure (a model of a crystal lattice is in Figure 3.16a). A pure crystal is often slightly lower in energy than an impure
Source Image: cambridge.org
Download Image
Solved orrect Question 10 0/0.5 pts Faster solid formation | Chegg.com Jan 30, 2023Recrystallization, also known as fractional crystallization, is a procedure for purifying an impure compound in a solvent. The method of purification is based on the principle that the solubility of most solids increases with increased temperature. This means that as temperature increases, the amount of solute that can be dissolved in a solvent

Source Image: chegg.com
Download Image
Fatty acid, triglyceride, and kinetic properties of milk fat fractions made by the combination of dry fractionation and short-pa Crystallization of a pure compound is spontaneous only below 109. °C. This reaction is exothermic and proceeds faster at temperatures above 34. °C. conclusions AH is AS is AH is AS is AH is AS is (pick one) (pick one) (pick one) (pick one) (pick one) (pick one) Question
Source Image: journalofdairyscience.org
Download Image
SOLVED: Use the observations about each chemical reaction in the table below to decide the sign (positive or negative) of the reaction enthalpy AH and reaction entropy AS, Note: if you have v t e Crystallization is the process by which solid forms, where the atoms or molecules are highly organized into a structure known as a crystal.
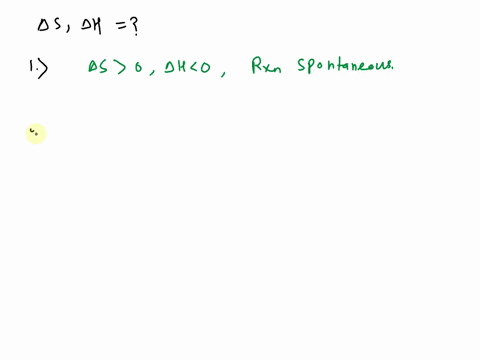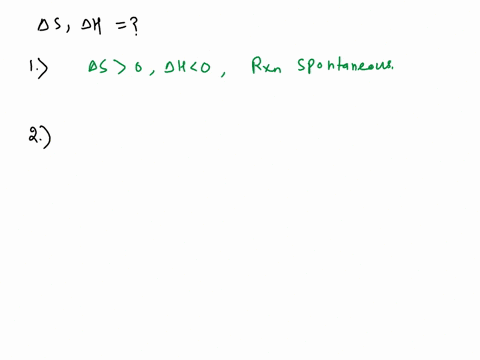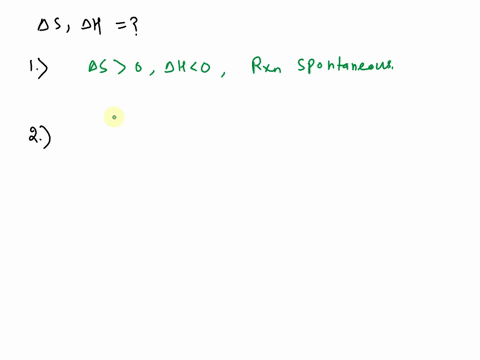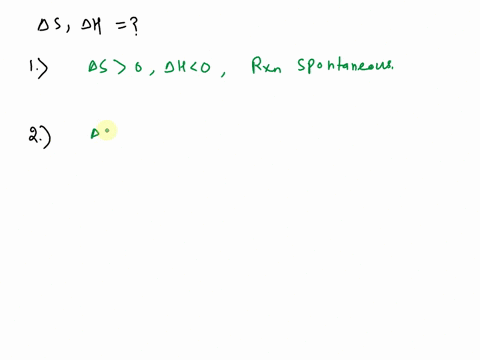
Source Image: numerade.com
Download Image
Crystal Growth, Thermal Treatment, and Characterization of Nonlinear Optical Crystal LiGa0.5In0.5Se2 for Mid-infrared Applications | Inorganic Chemistry A reactive crystallization could be performed for instance by adding a high pH solution to a low pH solution of a compound: the change in pH upon mixing the solutions reduces the solubility of the compound and crystallization can occur. A continuous reactive crystallization would have two solution feeds and a suspension outflow.

Source Image: pubs.acs.org
Download Image
IPI Volume 14 Issue 4 by Senglobal1 – Issuu Please also note that some compounds simpl crystallize more easily than others. More rigid molecules are, as a rule, easier to crystalize. 8 Rigid, in this context, mean compounds that contain fewer bond capable of undergoing internal rotation, so that there are fewer possible conformers possible. Let us go through a recrystallization process, focusing on technical aspects and trouble shooting.

Source Image: issuu.com
Download Image
Consumer Psychology of Individuals (Chapter 1) – The Cambridge Handbook of Consumer Psychology
IPI Volume 14 Issue 4 by Senglobal1 – Issuu Dec 17, 2023Countdown to Giveaway Crystallization Of A Pure Compound Is Spontaneous Only Below December 17, 2023 Question: What does a negative sign for delta G indicate at constant temperature and pressure? Answer: The reaction must be spontaneous ==================================================
Fatty acid, triglyceride, and kinetic properties of milk fat fractions made by the combination of dry fractionation and short-pa Crystal Growth, Thermal Treatment, and Characterization of Nonlinear Optical Crystal LiGa0.5In0.5Se2 for Mid-infrared Applications | Inorganic Chemistry v t e Crystallization is the process by which solid forms, where the atoms or molecules are highly organized into a structure known as a crystal.